INFLUENZA SURVEILLANCE LAB NETWORK
Backhe first outbreak of avian influenza virus A (February 2006) was reported in poultry in Nawapur district in Maharashtra with detection of H5N1 in the neighbouring district of Ucchal in Gujarat state. Since the first reported case of AI in poultry, the disease has been confirmed in Maharashtra, Gujarat, Madhya Pradesh, Manipur, Tripura, West Bengal, Jharkhand, Odisha etc. The World Bank was requested by the Department of Economic Affairs, Ministry of Finance on 19th January, 2006 to support India’s Country Programme for Preparedness, control and Containment of Avian Influenza. The Bank agreed to support and confirmed through a communication to DEA, dated 7th March, 2006 that the on-going IDSP would be primary vehicle for delivering such support. The Bank support focused on specified surveillance and diagnostic activities under the Country Programme and was divided as human health related activities and animal health related activities. The MOHFW & WB agreed that the costs of human health component will be met within the financial outlay available for the IDSP and funds for Avian Flu Component went directly to the central level labs/central autonomous institutions and not to states.
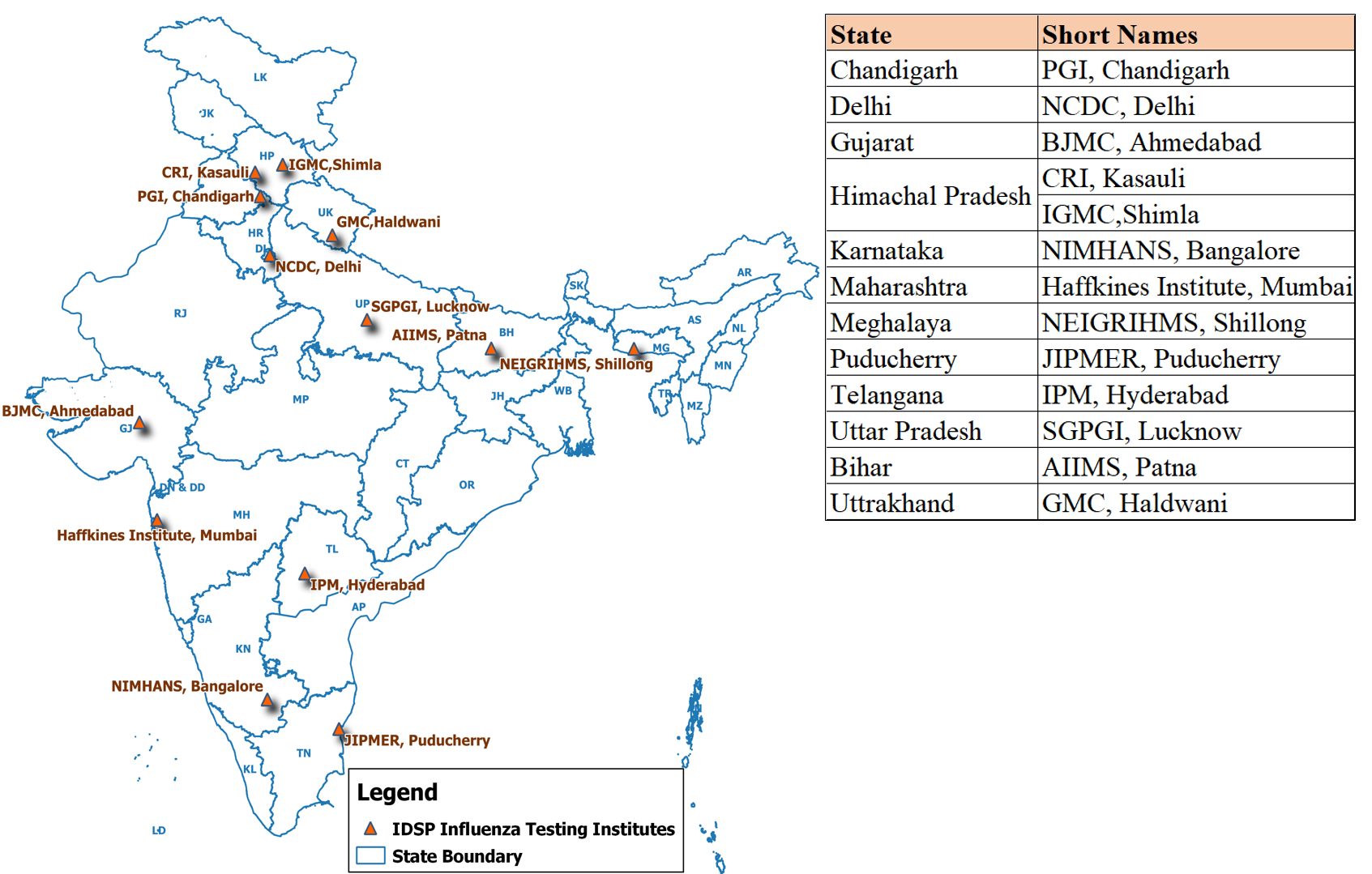
Human Health-The human health sub component of the AI component of IDSP was aimed to minimize the threat posed to humans by Highly Pathogenic Avian Influenza (AI) infection and other zoonoses and prepare for prevention, control and response to an influenza pandemic in humans. It supports: i) strengthening and networking of reference laboratories for prompt case confirmation: and ii) re-establishing seasonal influenza surveillance system for India. Initially the network included 12 Regional labs with the Microbiology Division at NCDC as the National Reference Laboratory. These labs are supported with the range of equipment required to carry out Real time PCR, funds for hiring a technician and for operational expenditure. In addition the commercial kits for real time PCR testing are supplied by the National Reference Laboratory at NCDC.
Influenza lab Network under IDSP:
| No | Name of Lab |
| 1 | Post Graduate Institute of Medical Education & Research, Chandigarh |
| 2 | National Centre of Disease Control, Delhi. |
| 3 | B.J. Medical College, Ahmedabad |
| 4 | Central Research Institute, Kasauli |
| 5 | Indira Gandhi Medical College, Shimla |
| 6 | NIMHANS, Bangalore |
| 7 | Haffkines Institute, Mumbai |
| 8 | NEIGRIHMS, Shillong |
| 9 | JIPMER, Puducherry |
| 10 | Institute of Preventive Medicine, Hyderabad |
| 11 | Sanjay Gandhi Post Graduate Institute, Lucknow |
| 12 | All india Institute of Medical Sciences and Research, Patna |
| 13 | Government Medical College, Haldwani |
The successes of the IDSP influenza networking during the recent H1N1 pandemic lies primarily in the fact that there was an established plan under the IDSP project to engage carefully selected laboratories with a strong central leadership provided by NCDC, Delhi. The MoUs under the IDSP had already been signed and the PCR machines supplied before the pandemic H1N1 (2009) was declared and with few additional inputs (training, startup consumables and kits), these laboratories could quickly gear up to verify the samples for influenza. Two rounds of training Programmes had been held for the nodal officers and the lab technicians from these labs, with the second training covering the H1N1 identification using real time PCR. NCDC in collaboration with WHO also developed a video on essentials of sample collection for influenza and Personal Protective Equipment which has been extensively used by the states for capacity building. A training toolkit for rapid response teams dealing with influenza also included important components of sample collection and PPE were developed by MOHFW jointly with WHO.
The project has played a crucial role in the containment of H1N1 pandemic through laboratory diagnosis and contact tracing using IDSP network. Some of the laboratories tested several thousand samples during the H1N1 pandemic for the respective states and also carried out operational research (including monitoring for antiviral resistance and evaluating other testing methodologies (rapid kits etc) for use in influenza diagnosis. The toll free call center 1075 was effectively used to inform medical personnel and general public about H1N1 including the location of nearest diagnostic centers.
Screening for swine flu at the Airports as a part of containment strategy

Training on Infection Control and Prevention

All AI laboratories were functional and assisting the respective states in influenza diagnosis. The scope of Avian Influenza laboratory network has been expanded to carry out sentinel surveillance for Influenza like illness (ILI) and Severe Acute Respiratory Infection (SARI) and monitor changes in the circulating influenza strain (if any). The plan included each regional lab to identify 3 sentinel sites and on weekly basis collect and analyse samples from ILI and SARI cases along with epidemiological information. A network meeting of the influenza laboratories in the country was conducted for experience sharing towards the goal of furthering influenza surveillance initiatives in the country and was planned in collaboration with the WHO India. The ultimate goal of the Influenza lab network is influenza surveillance in the country with a reasonable geographic representation.
There is need to expand the ongoing influenza surveillance in the country to make the data geographically representative and link disease surveillance at the field level with laboratory surveillance. Plans for including 4 more Regional labs are under process. In the larger context, the apex laboratory at NCDC needs to work towards becoming a National Influenza laboratory recognized by the WHO Global influenza surveillance network (GISN) and participate in Flunet so that the representative virus isolates from the country feed into the global influenza repository, based on which influenza vaccine composition is derived every year.
Animal Health
DADF has been able to successfully contain HPAI outbreaks in several States in India since the project became effective in 2007. India has been able to declare itself free of the disease, as a result. This has been possible in large part because of the improved capacity of DADF at all levels to detect and control H5N1 outbreaks. DADF surveillance capability was strengthened through the many workshops and training Programmes for vets, para-vets, Rapid Response Teams, villagers and field staff. By providing BSL laboratory facilities, the project has improved national diagnostic capability also. This clearly indicates that the animal health component of this project played a very important role in the containment of H5N1 and the security of the poultry industry in India.