LABORATORY STRENGTHENING
BackUnder Integrated Disease Surveillance Programme (IDSP) strengthening of public health labs is an important component which is mainly focussed on developing lab capacity in India such that states have the diagnostic facilities to conduct surveillance of epidemic prone diseases in a decentralized manner. The IDSP public health lab strengthening plan is currently based on two strategies:
1. Strengthening of district public health labs in the country in a phased manner
Laboratories at District hospitals are being strengthened for diagnosis of epidemic prone diseases with respect to deficient equipment, manpower and funds for consumables. In the year 2008-10, 50 district labs were taken up for strengthening across the country. In year 2010-11, plan to strengthen public health labs in all districts of the country in a phased manner was approved and communicated to the states.
The district public health labs under IDSP are expected to:
a) Perform testing for lab confirmation of epidemic prone diseases for both out patients and inpatients attending the hospital so as to generate lab confirmed surveillance data and
b) Support outbreak investigations in the district
c) Report weekly surveillance data on L forms.
These labs are expected to perform at least the following tests:
a) Blood culture for Typhoid
b) Stool culture for cholera and other common enteropathogens
c) IgM ELISA for Dengue (in endemic areas)
d) ELISA for Hepatitis A & E
e) ELISA / rapid test for Leptospirosis (in endemic areas)
f) Tests for other outbreak prone diseases prevalent locally
2. Referral lab network
Under this network, services of existing functional laboratories (at government medical colleges) are being used to allow access to quality laboratory services for investigation of outbreaks in the linked districts. A concept note was provided by CSU to the states for drafting the referral lab network plan. The plans were finalized through state level meetings with all the stake holders. The implementation process involves certification of labs by team of experts from the state followed by signing of MoU between SSU and referral labs and disbursement of annual grant to the referral labs. The referral labs are to be monitored through quarterly reports submitted by them to the SSU and the CSU (in prescribed format). Tests to be performed by the state referral labs are:
- Cholera and other enteropathogens : culture and sensitivity and serotyping
- Typhoid: Blood Culture & antimicrobial sensitivity (including isolate confirmation with specific antisera
- Bacterial meningitis-rapid latex agglutination test, CSF examination-wet mount, gram stain and culture & antimicrobial Sensitivity
- Hepatitis A&E- IgM ELISA
- Measles- IgM ELISA
- Dengue- IgM ELISA
- Leptospirosis-ELISA/Rapid test
- Diphtheria-Smear examination, culture and toxigenecity testing
- Any other tests for locally prevalent epidemic prone disease (to be identified by the state)
To ensure success of the implementation, the project has provided guidelines for various activities and assures continuous handholding at state and district level.
Another important aspect of IDSP is to strengthen reporting of laboratory confirmed surveillance data using the L form. All the referral labs under the lab network are also to report their weekly data using the L forms.
Assessment tool for Status of Laboratory component in States
The list of SRL s and the linked districts (as of March 2017)
Additional technical strengthening initiatives under IDSP
1. Assessment of laboratories:
IDSP conducted, with technical support from the CDC/Global Disease Detection –India Centre (GDD-IC), a standardized assessment of its public health laboratories and laboratory system. Assessments were conducted by using the standardized WHO Laboratory Assessment Tools (LAT), adapted to the Indian context. The goal of these assessments was to provide objective data so that IDSP can make evidence-based decisions on improving its laboratory capacity for disease detection, surveillance, outbreak response and also support the International Health Regulations 2005 (IHR) implementation in the country. A workshop was organized from 9 to 13 September 2013 at NCDC, Delhi for finalization of the tools for these assessments. This workshop was attended by Officers from CDC, GDD-I/C, NCDC and WHO. Eleven assessors were identified and trained at NCDC by experts from IQLS France and CDC Atlanta.

Figure: Training of Assessors for assessment of IDSP labs (conducted by CDC, Atlanta and IQLS France), at NCDC on 11-13 November 2013
In November 2013, after conducting a sensitization meeting for stakeholders from the states, the assessment was carried out in 19 IDSP district labs in 11 states. In addition, in 2 states assessment of representative laboratories at each tier of the laboratory network was conducted. The final gap analysis report of the assessments was presented by officers from IQLS and CDC in April 2014 during a meeting of state surveillance officers and state microbiologists held at NCDC. The data generated during these assessments formed the basis of a detailed gap analysis based on which activities for establishing LQMS in the IDSP labs have been initiated.
2. Laboratory Quality Management System (LQMS) training workshop for IDSP state Microbiologists. Under IDSP state microbiologists have been posted at State Surveillance Units to support the implementation of the laboratory strengthening activities of IDSP. A training workshop on LQMS was conducted from 17-20 November 2014 for IDSP state microbiologists to equip them with skills to strengthen quality management in IDSP laboratories.

Figure: Training workshop on LQMS for IDSP state microbiologists
3. Project on implementation of Laboratory Quality Management System in IDSP state referral labs in Assam and Punjab. A project on implementation of LQMS was initiated in the 4 IDSP SRL s (state referral laboratories) in Assam- Guwahati, Silchar, Barpeta and Jorhat, (Dibrugarh medical college is already NABL accredited) and Punjab – Patiala, Faridkot, Ludhiana and Amritsar. During 9 months of the project from October 2015 to June 2016, as a first step the labs identified a Quality manager, subsequently a baseline assessment was done using the WHO s Laboratory Quality Stepwise Implementation (LQSI) Tool checklist, with focus on phase 1 and phase 2 of the tool. Based on gaps identified, specific lab based action plans were made and 2 trainings were held on the strengthening of various quality essentials including equipment and inventory management, Quality control, Biosafety. The pilot ended in June 2016 with NABL internal auditor training of the quality managers from these labs.
4. Pilot Project on strengthening of Food Borne and Acute Diarrheal Disease surveillance and outbreak response:
Acute diarrheal diseases (ADD) and food poisoning account for 46% of disease outbreaks reported to the Integrated Disease Surveillance Program (IDSP) in India. However, district-level investigations frequently lack thorough epidemiologic and microbiological evaluation.
To address this, NCDC, with technical support from CDC/GDD-IC, initiated a pilot project on Acute Diarrheal Disease/Food borne Disease surveillance to enhance the epidemiologic and laboratory detection and response to foodborne and ADD outbreaks through the IDSP framework in 2 states- Tamil Nadu and Gujarat. The overall vision for the project is to strengthen a coordinated epidemiologic and laboratory response for foodborne and ADD outbreaks, as outlined in Figure below. Illness that is detected at the sub-district level is reported to district officials. The block and district rapid response teams are triggered to initiate a coordinated response: from specimen collection, processing, and pathogen identification on the laboratory side, to outbreak confirmation, field investigation and implication of the responsible food or water vehicle on the epidemiologic side. The data gathered can be used to control and prevent further infections and potentially also future outbreaks.
The broad goals of the pilot project are to strengthen pre-analytic, analytic, and post-analytic components of laboratory investigation of ADD and foodborne disease outbreaks, build district and state-level capacity for more systematic and analytically driven epidemiologic investigation of outbreaks, and initiate and strengthen the capacity for routine laboratory based ADD surveillance within the IDSP framework.
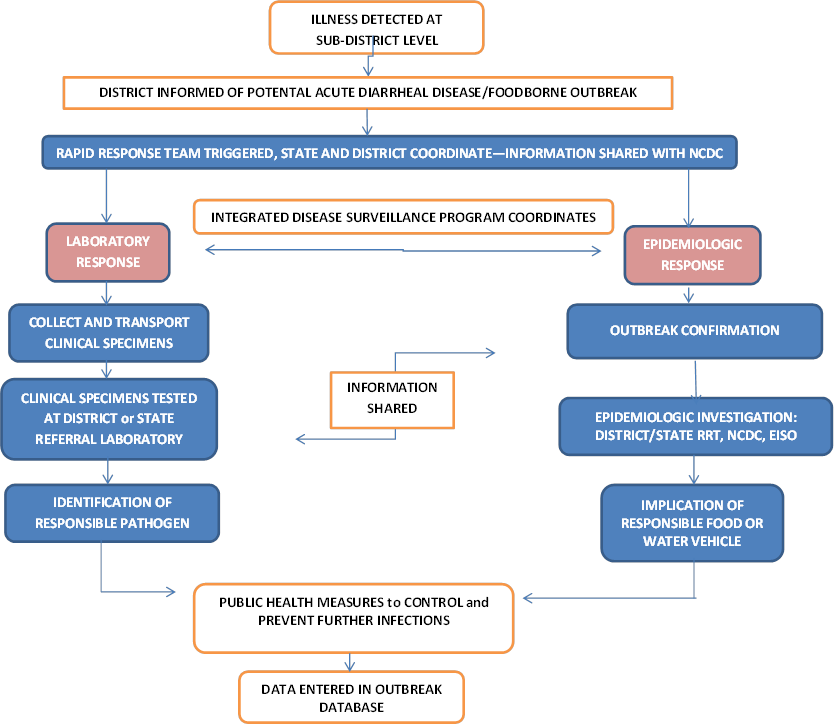
Figure: Coordinated Epidemiologic and Laboratory Response to Foodborne and ADD Outbreaks
The finalized formats, log books, and templates developed for epidemiologic and laboratory investigation of ADD and foodborne disease outbreaks in the pilot districts has been compiled to form an “IDSP ADD/Foodborne District Level Outbreak Investigation Toolkit” for the systematic investigation of foodborne and acute diarrheal disease outbreaks at the district level and have been uploaded on the IDSP portal. This toolkit will support the expansion of the pilot to other districts in Tamil Nadu and Gujarat, and subsequently to other states in a phased manner. Furthermore, the initiation of routine laboratory-based surveillance for ADD can be extended to other districts and pathogens, and form a basis for enhanced outbreak detection as well as begin to contribute toward a better understanding of diarrheal disease pathogens and burden in sentinel areas across the country.
IDSP ADD/Foodborne District Level Outbreak Investigation Toolkit
1. Action points for epidemiological investigation of suspected foodborne/ADD outbreak
ADD/FBD Pilot Project Activities until July 2016
Historical background
Laboratory Strengthening under IDSP aimed to upgrade laboratories at all levels, from peripheral to national reference laboratories, and introduce a quality assurance system for assessing and improving quality of laboratory data. However, implementation experience and limited availability and capacity of staff proved that it was not possible to expand the laboratory strengthening as expected. As a consequence, the laboratory strengthening component was revised several times, leading to confusion at state level and affecting the implementation.
As per initial PIP, the IDSP envisaged upgrading of laboratories to provide rapid and reliable confirmation of suspected cases, monitoring drug resistance and monitoring changes in disease agents and introduction of laboratory quality assurance systems. In order to allow rapid and reliable laboratory confirmation of causative agent, as well as to improve the quality of laboratory data, a five level laboratory network was planned under IDSP which included: Peripheral Laboratories and Microscopic Centers (L1 labs); District Public Health Laboratories (L2 Labs); Disease Specific State Laboratory (L3 Labs); Regional Laboratories (L4 Labs) and National Reference Laboratories (L5 Labs).
L1 laboratories were to provide information for the diagnosis of malaria, TB, typhoid, chlorination levels in water and fecal contamination of water. Whilst these laboratories already handled examination of sputum and blood smears, some needed internal modification as well as the provision of kits for typhoid diagnosis and assessment of fecal contamination of water. L2 laboratories were needed to carry out tests for TB, malaria, typhoid, cholera and water quality, primarily to confirm results from the peripheral levels and for quality control. Some required internal modification and additional equipment, reagents and kits. These laboratories were to be connected to the computer network. L3 laboratories were to carry out tests to confirm L1 and L2 results, as part of the internal quality control mechanism, and assays required for the non-communicable disease surveys. The project provided for internal modification of laboratories, equipment required for additional tests, reagents and kits, and a computer, software and telephone connectivity. L4 laboratories identified under IDSP were to have one central and four regional laboratories to support routine work and specific outbreak investigations. These were high quality laboratories which already had the capacity to carry out the laboratory tests required. The project was to fund the incremental operating costs and, if required, a computer, software and telephone connectivity. L5 laboratories - Disease based national reference Laboratories. These laboratories were to provide support for disease specific requirements like virus isolation, sero-typing etc. and play active role in promoting quality assurance and test kit standards.
Further discussions with states suggested that many Primary/Community health centre (Level 1) laboratories had already been renovated under Revised National TB control and Enhanced Malaria Control projects. Therefore to rationalize the resources provided under the project for laboratory renovation it was recommended to address critical needs of District Public Health (Level 2) laboratories. States carried out an extensive assessment of the deficiencies in the district labs. Based on this data from the states the consignee list was prepared and basic equipment was procured centrally and supplied to more than 200 districts in 9 phase 1 states through the procurement agency HSCC. A training Programme was also developed for laboratory staff at all levels.
A baseline survey was carried out in these 9 phase 1 states by hiring an external agency. The key finding of this survey was that most of the districts do not have microbiologists and some of them do not even have a qualified doctor or technician to operate a lab. Even in places where staff was available, there had been no training. The limited availability and capacity of staff proved that it was not possible to expand the laboratory strengthening as envisaged under the project. It was realized that the task of supporting more than 600 district labs was too challenging due to limited availability of microbiologists. Hence the procurement of equipment for the phase 2 and phase 3 states was stopped.
Based on the findings of baseline survey and implementation experiences, IDSP revised the plan for laboratory strengthening. Following a detailed state by state situational analysis of the laboratory component, concept of developing 50 model public health labs in different geographical areas in the country evolved. Under the project these labs were to undertake diagnostic tests for common epidemic prone diseases.
By end of the year 2008, a clear concept of two pronged approach emerged.
a) Strengthening of 50 district priority labs (DPLs) all over the country with the objective to generate lab confirmed quality surveillance data for common epidemic prone diseases at the district level and lab investigation of outbreaks.
b) Establish a network of referral laboratories utilizing the services of existing functional laboratories in the states. In the light of severe human resource limitations, instead of creating new infrastructure, it was decided to partner with existing functional laboratories at government medical colleges and other large institutions for the etiological diagnosis of outbreaks. This network would allow the linked districts to have access to quality public health laboratory services for outbreak investigations. An output based arrangement would be used for involving these labs in disease surveillance. The lab networking in 7 priority states on a pilot basis was planned.
Fifty DPLs were planned to be strengthened according to Indian Public Health Standards, 2 District labs each from 14 focus states and one largest state Uttar Pradesh and 1 each from other 20 states/UTs. Model plan was made for District Priority laboratories. Tests to be done by these labs for etiological diagnosis of common epidemic prone diseases were identified. Performa for survey of district priority labs were drafted and sent to states. Most states did not have dedicated public health laboratories and public health services were provided by multiple laboratories. Few states (Karnataka, Maharashtra and Gujarat) which did have dedicated public health labs, the services of these labs were mostly limited to undertaking water analysis. Thus the clinical lab attached to the district hospital was proposed for strengthening under IDSP. This approach would also establish a mechanism for generation of laboratory based routine surveillance data of epidemic prone diseases in the districts.
The states identified the district labs and sent the duly filled in survey forms to the CSU. Detailed analysis of survey forms sent by the states for the identified priority laboratories was done at CSU. Deficiencies in terms of essential equipment were identified for these labs. No objection certificate for state level procurement of these equipment was obtained from the World Bank. Technical specifications of the equipment to be procured were made and approved by an expert group constituted under the Ministry of Health and Family Welfare (MoHFW). Approval was obtained from MoHFW for state level procurement of specific equipment for the 50 DPLs. Procurement guidelines for state level procurement of equipment were firmed up and approved by World Bank. Communication was sent to the states for procurement of equipment. The strengthening plan of 50 district public health laboratories continued to be hampered by limited human resources available at district and central level, as well as limited insight at state level about the necessity of laboratory confirmation during outbreak investigations and importance of laboratory confirmed surveillance data at district level.
Towards establishing the referral lab network, the concept note was prepared including the financial details and shared with the World Bank. Subsequently an MoU was developed after discussions with the World Bank. The MoU was to be signed between the state surveillance units and the identified referral labs. State level sensitization meetings were held with various stakeholders and state referral lab network plans were drafted. The officers from World Bank and CSU, IDSP participated in these meetings. The plans drafted in these meetings were subsequently approved by the state health societies.
The laboratory component under the restructured project for year 2010-12 included the two pronged approach of strengthening 17 district level public health labs and to establish the state referral lab network in the 9 World Bank funded states. The remaining 33 district public health labs in 26 states were funded under the domestic budget. The district public health labs were supported under the project by provision of a trained microbiologist and funds for purchase of consumables.
During 2010-11, in 9 States (Gujarat, Punjab, Rajasthan, Uttarakhand, Karnataka, Tamil Nadu, Maharashtra, Andhra Pradesh and West Bengal), the state referral lab network involving 65 Institutions was established and 222 suspected outbreaks were investigated by these institutions in the year 2011. During the 12th Five Year Plan, in year 2012-13 additional 23 identified medical college labs in Bihar, Assam, Odisha, Tripura, Kerala, Haryana, Jammu & Kashmir and Manipur were included in the laboratory network. In the year 2013-14, state referral lab network involving 8 additional institutes has been established in 3 states namely Jharkhand, Chhattisgarh and Madhya Pradesh. (Annex 6: List of Institutions under the State Referral Lab Network under IDSP). A total of 601 suspected outbreaks were investigated in the year 2012 by this network, 920 in the year 2013 and as of June 2014, 345 suspected outbreaks have been investigated by the Institutions under the state referral lab network of IDSP.
The strengthening plan of 50 DPLs continued to be hampered due to limited insight at state level about the importance of laboratory confirmed surveillance data and necessity of laboratory confirmation during outbreak investigations. During field visits it was further realised that the DPLs are unable to function optimally due to lack of support staff in the lab that is technicians and attendants. Some other hurdles in making these labs functional were lack of integration of the IDSP labs with the district hospital laboratory. While the patient walking in the district hospital was not getting investigated for the common epidemic prone diseases and was being managed syndromically, there was this stand-alone IDSP lab in the same district with reagents and microbiologist waiting for samples from the field. These stand-alone IDSP labs in the districts were unable to fulfill the key objective of lab strengthening under the project that is to generate lab confirmed surveillance data for epidemic prone diseases at the district level.
With the growing importance of laboratory strengthening for public health in the country, at a meeting taken by the then Secretary Health (H&FW) on 9 November 2010, NPO IDSP was asked to prepare a plan for developing one lab per district for public health network. A concept note was prepared for establishment of public health labs at district hospitals (DPHLs) in all districts in the country in a phased manner. This concept note addressed the various gaps which were identified during the lab strengthening experience under IDSP. A communication regarding the same was sent in December 2010 by the then MD NRHM, Govt. of India to the states to take up development of the district laboratories for diagnosis of epidemic prone diseases in a phased manner. The support for these labs included funds for all the basic equipment required for a microbiology lab, funds for consumables, kits and reagents and manpower including microbiologist, technicians, lab assistant and lab attendant. The states were to initiate this lab development in the districts where such facilities are not available. Based on the initial survey of the identified labs against standard equipment and manpower proposed in the concept note, a budget for gaps in equipment and manpower was to be prepared by the states. Cost of kits and reagents was to be budgeted as per the number of tests expected to be carried out per annum. These labs were to be budgeted under the NRHM flexipool head in the PIP for year 2011-12
During the 12th Five Year Plan, this establishment of public health labs at district hospitals has been incorporated in the IDSP budget with some modifications in the manpower provisions. By year 2017, a total of 300 District Public Health Labs (including the initial 50 labs) are to be functional for diagnosis of common epidemic prone diseases and must generate lab confirmed surveillance data for common epidemic prone diseases and carry out lab investigation of the outbreaks. As of September 2014, a total of 145 district labs in 23 states have been approved under IDSP for development. However, for successful functioning of these labs, it is crucial that these district laboratories are managed by qualified microbiologists. State ownership must be demonstrated by mobilizing adequate samples, making provision for appropriate consumables and supplies, supplementing support provided by IDSP and human samples get priority over environmental samples.
Under IDSP, various guidelines and training materials have been developed. A Bio-safety manual was developed in the year 2007-08 focusing on Infection Management, worker safety and waste management, to be a reference guide for all levels of laboratories. Training material for Induction training of Microbiologists under IDSP, covering standard operating procedures, internal quality assurance, biosafety and biomedical waste management issues, was developed in the form of Laboratory Manual which was later revised with the support of WHO and uploaded on the IDSP web site in the year 2011. Guidelines for adequate specimen transportation and collection during outbreaks at the district level have also been communicated to the states.
A meeting of Nodal Microbiologists from 65 referral laboratories, State Lab Coordinators and State Microbiologists under IDSP was organized on 8-9 December 2011 at NCDC, Delhi. The objective of the workshop was to sensitise the microbiologists to International Health Regulations (IHR) 2005, monitor the working of the state referral lab network and to share their success stories and the challenges faced.
Under MoHFW, a National Laboratory Expert Group was constituted to guide and assist IDSP in strengthening of district public health labs in the country. The first meeting of this expert group was held at NCDC on 18-19 October 2011 with the objective to prepare a roadmap for establishing and strengthening of public health laboratories under IDSP and to develop the action plan for establishing national level laboratory network for disease of public health importance including outbreak investigation of unknown etiology.
External Quality Assurance Scheme (EQAS)
As per the action plan of the World Bank funded restructured project, one of the key activities towards monitoring the functionality of the labs was by their participation in National External Quality Assurance Scheme (EQAS). The EQAS programme was initiated for 17 DPLs and all the 65 labs under the State Referral Lab Network in the 9 World Bank funded States. The EQAS was conducted by National Centre for Disease Control (NCDC). Each panel had a pair of identical slides for gram staining; and two agar stab cultures for isolate identification, serology and antibiotic sensitivity testing. Each participating lab was identified by a unique confidential code. These sets were randomly processed in two of the diagnostic labs at NCDC as Internal Quality Controls. The results of the Internal Quality Control Panels were evaluated and were found to be correct. These panels were distributed to the participants during the two days Workshop held in December 2011 at NCDC organized for the Nodal Microbiologists of the 9 World Bank Funded states.
A Performa stating the protocol for processing the samples and a reporting format was given along with the panels to the participating laboratories. A specified time frame of 15 days was also given to each lab for reporting the results. The participating labs shared the EQAS results with IDSP and feedback report regarding their results was sent to them as soon as the evaluation process was completed.
In all, 92.7% (n=76) labs reported their EQAS results. Of 76 labs, 67.1% (n=51) labs attained >85% grades and their performance rated as Excellent. As per results of the EQAS conducted in December 2011, 90.2% of state referral labs and 33.3 % of District Priority labs are properly equipped to perform the various laboratory tests.